Here's why a lithium battery degrades and loses capacity over time

Lithium-based batteries – the kind of which are used in pretty much every modern cell phone – don't last forever. About a fifth of a cell's charge capacity is lost by the time its 500th charge cycle is reached, and, unfortunately, there's nothing you can do about it. Chances are, however, that one day we will have more durable, longer-lasting batteries inside our gadgets, and a recent study conducted by the Pacific Northwest National Laboratory may make this day arrive sooner.
Using a powerful microscope, a team of scientists at the lab have made it possible to observe a lithium battery in real time as it charged and discharged. What they found was that the stress caused by the battery being used was causing cracks to develop on the electrode. This, however, is just one of the reasons why a lithium battery's performance decreases over time. As the research showed, each charge and discharge cycle leaves traces of lithium outside of the cell's electrode. This "dead" lithium, as the scientists refer to it, could not contribute to a future charge and was causing the battery's energy capacity to diminish. Furthermore, it was observed how a layer of solid-electrolyte interphase formed on the electrode's surface, thus "clogging" the battery and hindering its ability to take charge.
Using alternatives to lithium could be a solution to the problem. Metals like magnesium, aluminium, or copper could one day serve as a substitute that is both cheaper and more reliable, but at this time, more research into non-lithium rechargeable batteries is needed if they're ever to reach the performance of existing lithium-based solutions.
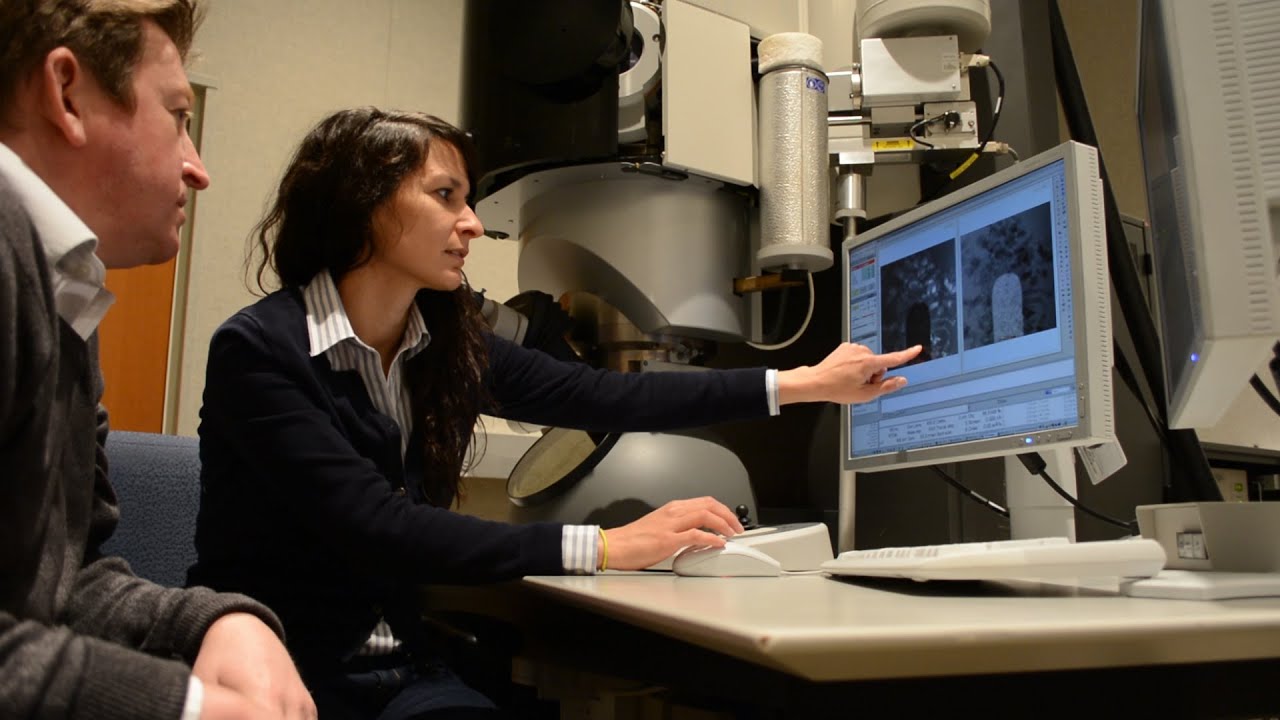
source: Pacific Northwest National Laboratory (Cached version)
Battery-related articles you might also like:
Follow us on Google News









![Some T-Mobile users might be paying more starting in March [UPDATED]](https://m-cdn.phonearena.com/images/article/176781-wide-two_350/Some-T-Mobile-users-might-be-paying-more-starting-in-March-UPDATED.webp)











Things that are NOT allowed:
To help keep our community safe and free from spam, we apply temporary limits to newly created accounts: